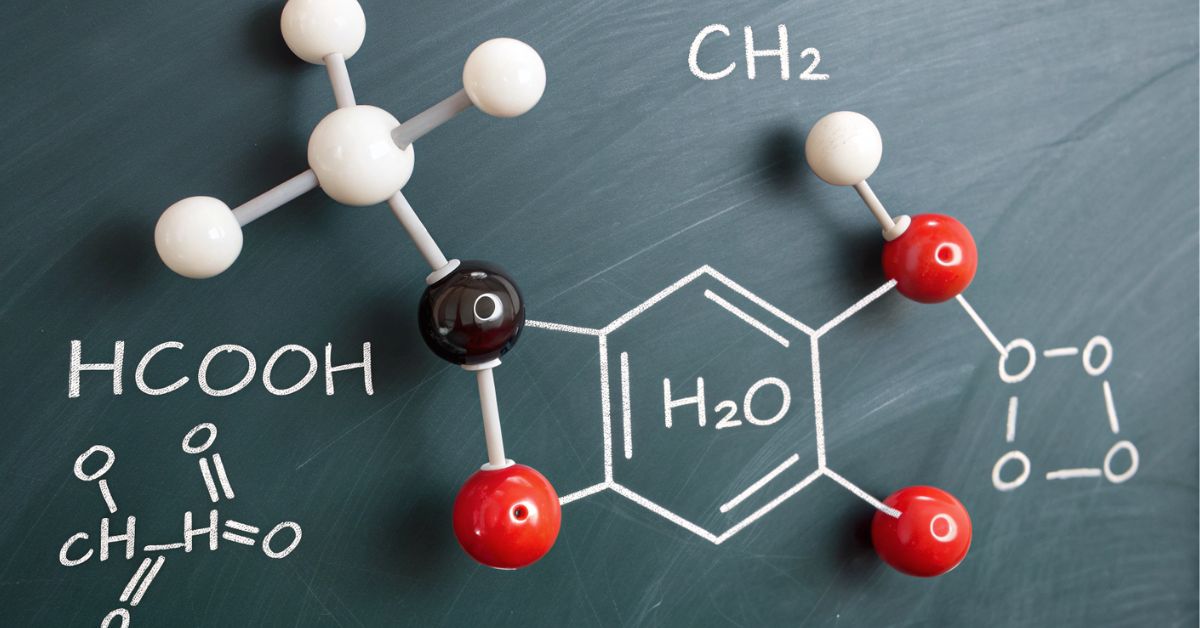
Methyl formate (chemical formula HCOOCH₃) is the simplest ester derived from formic acid and methanol.
When it reacts with water (H₂O), it undergoes ester hydrolysis—a fundamental chemical reaction taught in chemistry classes and used widely in industry. This process produces two important substances: formic acid (HCOOH) and methanol (CH₃OH) (unsentmessageproject.com, talkingcity.com). Understanding this reaction helps students learn how esters break down in water under acidic or basic conditions, https://www.tanzohubs.com/markiseteppe-stylish-durable-shade-ground-cover-for-outdoor-living/ and it also lays the groundwork for more complex processes like soap-making or biodiesel production.
In this article, we will explore what methyl formate is, the chemistry behind its reaction with water, how it’s used, pros and challenges, answers to common questions, and why it matters.
What Is Methyl Formate?
Names & Formula:
Methyl formate, also known by its systematic name methyl methanoate, has the chemical formula HCOOCH₃. It is the simplest ester formed from the combination of formic acid (HCOOH) and methanol (CH₃OH). It belongs to a class of organic compounds called carboxylic acid esters, which are widely used in both industry and laboratories. In various scientific databases and educational sources like Wikipedia and Unsent Message Project, it is consistently referred to using this structure due to its widespread importance in organic chemistry.
Properties:
Methyl formate is a clear, colorless liquid with a distinct sweet, fruity odor that resembles rum or apples. It has a boiling point of about 32 degrees Celsius (90°F), which means it is very volatile at room temperature and evaporates quickly. It has a relatively low molecular weight of 60.05 g/mol, making it lightweight and easily diffused. It is slightly soluble in water, with about 30% solubility at room temperature, which is sufficient for many reactions but still allows it to separate from water in some mixtures. Due to its high vapor pressure and flammability, it must be handled with care in ventilated areas.
Uses:
Methyl formate plays an essential role as a chemical intermediate. It is used in the production of formic acid, which has applications in agriculture (as a preservative), textiles (for dyeing and finishing), and leather tanning. It is also used to produce methanol, an industrial alcohol that serves as a solvent, fuel, and antifreeze agent. In organic synthesis, methyl formate can also be used to create formamides, esters, and other derivatives. Additionally, due to its pleasant odor, it has seen limited use in fragrance formulation and as a blowing agent in foam production.
The Hydrolysis Reaction: Overview
When methyl formate meets water, the ester bond breaks and yields two products:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
- HCOOCH₃ = methyl formate (ester)
- H₂O = water
- HCOOH = formic acid
- CH₃OH = methanol (unsentmessageproject.com)
This reaction can occur slowly on its own or be sped up using acid (like HCl) or base (like NaOH) catalysts.
Acid-Catalyzed Hydrolysis
- Protonation: Ester’s carbonyl group is protonated, making it more reactive.
- Nucleophilic attack: Water attacks the carbonyl carbon.
- Tetrahedral intermediate: A short-lived structure forms.
- Leaving group departure: Methanol is ejected.
- Deprotonation: Formic acid is formed; catalyst is regenerated (unsentmessageproject.com).
Base-Catalyzed Hydrolysis (Saponification)
- Hydroxide attacks: OH⁻ attacks the carbonyl carbon.
- Intermediate forms: A tetrahedral transition state appears.
- Methanol leaves: CH₃O⁻ leaves as methanol.
- Formate ion forms: A formate salt appears (e.g., sodium formate if NaOH is used), which can be acidified to make formic acid.
Why It Matters (Applications)?
- Formic acid production: A simple, economical route to produce formic acid (talkingcity.com, unsentmessageproject.com).
- Methanol recovery: Methanol is a key solvent and industrial feedstock.
- Teaching tool: Excellent for demonstrating ester hydrolysis mechanisms in education (unsentmessageproject.com).
- Industrial relevance: Ester hydrolysis is key in soap-making, biodiesel, food additives, and biodegradable polymers.
Reaction Conditions & Factors
- Catalyst: Acid speeds up ester protonation; base creates irreversible saponification (unsentmessageproject.com).
- Temperature: Higher heat accelerates the reaction, but extremes may cause side reactions.
- Water amount: Excess water pushes equilibrium toward products (talkingcity.com).
- Pressure: Industrial setups may use pressure to improve contact and conversion.
Physical & Environmental Notes
- Solubility: Methyl formate is somewhat water-miscible; smaller esters are more soluble (Chemistry LibreTexts).
- Hazards: Highly flammable and volatile; vapors can irritate skin and eyes (CAMEO Chemicals).
- Environmental impact: Short-lived in the atmosphere and less damaging than some alternatives, but spills must be managed (CAMEO Chemicals, Wikipedia).
Industrial Catalysis (Advanced Topic)
Recent research explores manganese-based catalysts https://en.wikipedia.org/wiki/Catalysis to help dehydrogenate methyl formate in water, enhancing sustainability (Wiley Online Library).
Studies also examine how water content, temperature, and time affect yield and efficiency (ScienceDirect).
FAQs
1. What does HCOOCH₃ + H₂O produce?
It produces formic acid (HCOOH) and methanol (CH₃OH) (unsentmessageproject.com).
2. Can the reaction happen without a catalyst?
Yes, but it’s slow. Catalysts (acid or base) are used for faster and more efficient reactions (unsentmessageproject.com).
3. Is the reaction reversible?
Yes under acidic conditions, but base-catalyzed hydrolysis leads to irreversible saponification (unsentmessageproject.com).
4. What are industrial uses?
Primarily in formic acid and methanol production, as well as in teaching labs and soap/biodiesel manufacturing.
5. Is methyl formate safe?
Handle with care—it’s flammable, and its vapors can irritate. Use proper ventilation and protective gear (unsentmessageproject.com).
6. How do temperature and water affect it?
Higher temperature and excess water speed up the reaction and favor product formation (talkingcity.com).
7. Can enzymes break ester bonds like this?
Yes—biological enzymes like esterases/hydrolases can cleave similar ester bonds in living systems.
8. Why is it important to study this reaction?
It forms the foundation for understanding ester chemistry, vital in biochemistry and industrial processes (unsentmessageproject.com).
9. Does it happen in nature?
Not exactly methyl formate, but ester hydrolysis is common in digestion, metabolic pathways, and environmental breakdown.
10. Can the products be reused?
Yes—methanol is a solvent fuel, and formic acid is used in leather tanning, preserving, and textile processing.
Conclusion
The hydrolysis of methyl formate (HCOOCH₃ + H₂O → HCOOH + CH₃OH) is a key reaction in chemistry. It shows how esters break down in water, both in the lab and industry.
Whether catalyzed by acid or base, the transformation is essential for producing formic acid and methanol—important chemicals in everyday products. This reaction also forms https://www.tanzohubs.com/gobluecc-what-it-is-how-it-works-is-it-safe/ a foundation for understanding more complex organic processes. Safety considerations are important, as methyl formate is flammable and its vapors hazardous.
Overall, mastering this simple reaction opens the door to learning broader topics like saponification, biodiesel production, and environmental chemistry.